Time Medical Exhibited at the 108th RSNA and Held a Launching Ceremony on Neonatal MRI
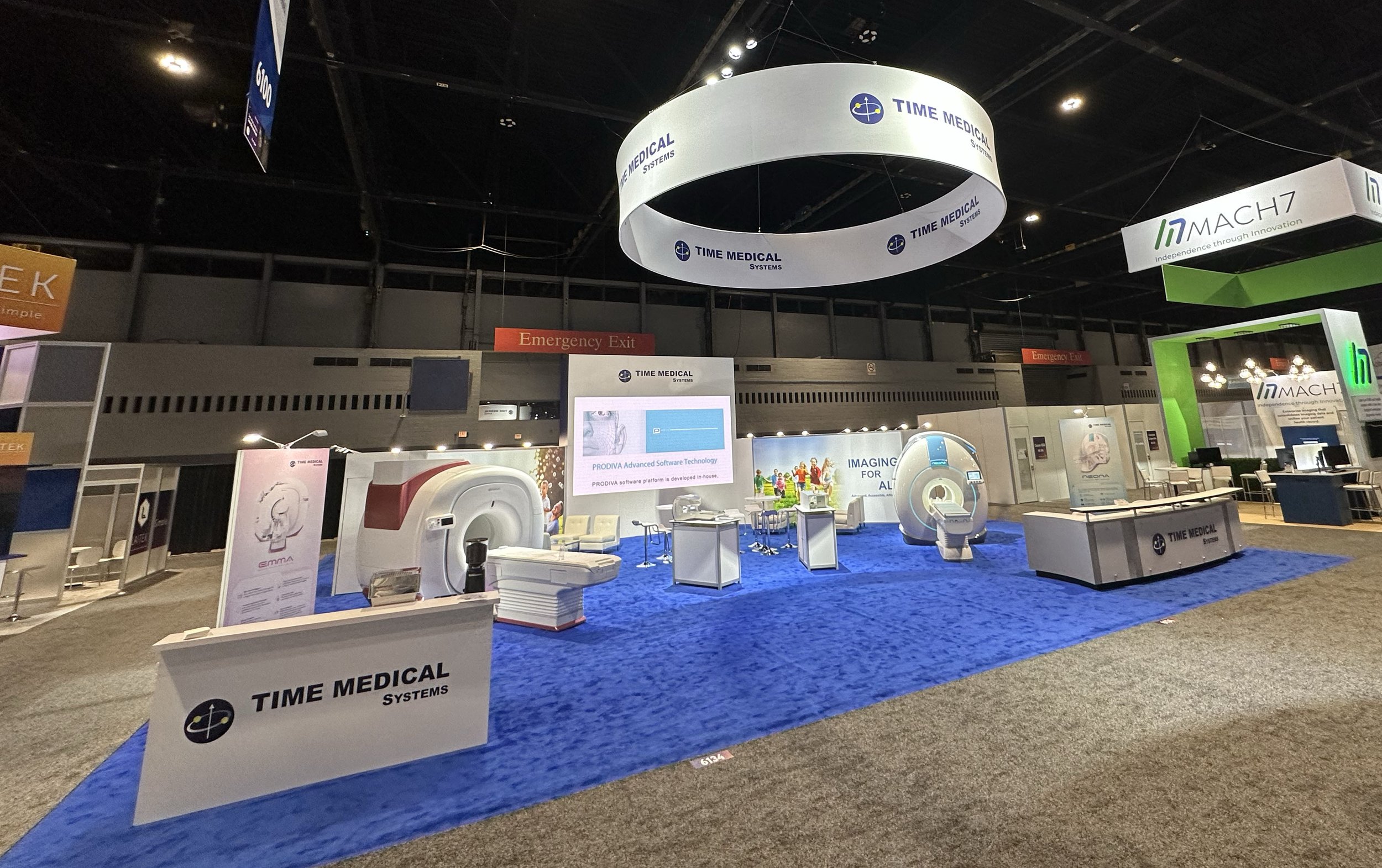
/Nov 27th - Dec 1st : The Radiological Society of North America’s 108th Scientific Assembly and Annual Meeting (RSNA) is an annual event held in Chicago. It is the largest and most influential international radiology industry conference and exhibition with more than 650 companies participating. Time Medical (TM) is proud to exhibit in the largest booth in its history, showcasing the company’s innovative Neonatal MRI and Breast MRI.
Time Medical’s booth at the 108th RSNA
The highlight of RSNA this year was AI and breast cancer screening. There were numerous special reports on AI from about a hundred companies participated in this field. Prof. Elizabeth Morris, Chair of the Department of Radiology at the University of California, Davis (UCD) and a recognized expert in breast imaging, delivered the first keynote report of the conference on "Saving More Lives". She pointed out that while breast MR is an effective method for the diagnosis of dense breast, only 2% of women in the United States undergo breast MR screening and most of them do not have regular checkups. Prof. Morris shared her personal experience that although she do mammography and ultrasound breast cancer screening test regularly, during her health checkup early this year, she received a breast cancer diagnosis that were not detected earlier. Her 4mm invasive lobular tumor was found only after tissue had been removed from her breast during surgery. As such, Prof. Morris strongly request for radiologists to advocate breast cancer screenings by MRI to save more lives. "If we can screen for all kinds of cancers early and treat them with minor surgery at an early stage, cancer will become a common disease rather than a fatal one." said Prof. Morris.
During the exhibition, TM sales team hosted more than 100 distributors and potential clients from more than 16 countries including the United States, the United Kingdom, Germany, Portugal, Sweden, Canada, Australia, Argentina, Chile, Peru, Mexico, India, Saudi Arabia, Egypt, Thailand, and South Korea. The team connected with more than a dozen American hospitals and imaging diagnosis chain companies, whom showed strong interest in TM’s breast MRI as an essential modality for early and accurate screening of breast cancer. On the other hand, TM’s neonatal MRI also attracted much attention from various hospitals’ representatives, including Massachusetts General Hospital of Harvard Medical School, Three of top 10 US Children Hospitals (Cincinnati Children Hospital, Stanford Children's Hospital, Colorado Children's Hospital), as well as Cambridge University School of Medicine in the UK, Children Memorial Health in Poland and Children's Health Ireland.
TM executives also met several industrial executives during RSNA, including the CEO of Cannon Medical, the CEO of Siemens MRI Division, the GM of Philips MRI Division, the CEO of Mardamed, the chairman of UAE BPG and the CEO of Cura India etc. to discuss collaboration plans. Mr. Toshio Takiguchi, CEO of Canon Medical Systems visited TM booth to view the Neonatal MRI, and had a meeting with Prof. Ma for potential collaboration in Asia market. Cannon is No. 1 player in Asia medical imaging market and No. 4 globally.
Canon Medical CEO Toshio Takiguchi and Prof. Ma
Time Medical officially launched its neonatal MRI (a.k.a. NEONA) on Nov 29, 2022. NEONA is the world's first superconducting MRI dedicated to infants, which greatly improves the quality of the diagnosis of newborns. This system was honored with the "Geneva Prize" at the Geneva Invention Congress. TM’s NEONA is light, fast, accurate and safe for it to be installed in the Neonatal Intensive Care Unit (NICU) for precise and radiation-free diagnosis of infants. Currently there are more than 4,000 NICUs across Europe & US as well as more than 3,000 children's hospitals in Asia.
The NEONA launch ceremony started with Prof. Ma giving the product introduction and summarizing its features into the tagline: "Small and light, fast and safe". The innovation of NEONA started with the suggestion from 2 top MRI experts - Professor Bradley and Mr. Michael Harsh. After years of research and development, the prototype of NEONA was completed in 2019. Affected by the COVID pandemic, the FDA application was delayed until July 2022. Today Time Medical's favorite “child” - NEONA, is finally born!
TM advisor and former GE Healthcare CTO Michael Harsh congratulated the birth of NEONA! He said: "Today, the industry finally realized the importance of specialized MR, which will open up new markets. I am very happy that TM has made the specialized MR a reality!" Everyone celebrated with ribbon cutting and champagne popping.
The VIP guests who participated in the event included the chair of the Department of Radiology at Mayo Clinic (the largest hospital group in the US) and former chairman of RSNA, Professor Richard Ehman; former CEO of GE Healthcare MRI Division Mr. Richard Hausmann; Professor Michael Gee, deputy director of the Department of Radiology at Massachusetts General Hospital of Harvard Medical School; Professor Wang Yi, a radiology expert from Cornell University School of Medicine; Mr. Anil Jain, GM of Refex Group in India; Mr. Bala, CEO of Cura India; and distributors from Europe and the US, etc.
Mr. Michael Harsh delivered a speech at the NEONA launching ceremony
Dr. Yuri Wedmid, Prof. Ma, Prof. Ehman, Mr. Richard Hausmann and Mr. Michael Harsh cutting ribbon
Prof. Garry Gold, head of the Department of Radiology at Stanford Medical School congratulated the team on the launch of NEONA
Yuri Wedmid, Michael Harsh, Michael Gee, Q.Y. Ma, Richard Hausmann and Richard Ehman
Prof. Brian Coley, Radiologist-in-Chief of Cincinnati Children’s Hospital congratulated TM
Yuri Wedmid, Anil Jain, Michael Harsh, Bala and Q. Y. Ma
CIBR (Coalition for Imaging and Bioengineering Research) held a meeting of the Steering Committee where Prof. Ma and Yuri were invited to attend. Dr. Renee Wegrzyn, the inaugural Director of ARPA-H (Advanced Research Projects Agency for Health), a newly established interdepartmental funding agency in the US were invited to introduce the functions and goals of APRA-H. She pointed out that the agency is different from the National Institutes of Health (NIH) and will mainly focus on high-risk, cutting-edge research and development projects in life and healthcare space such as how to prevent people from becoming patient. The agency will start operation early next year and expects active participation from CIBR members. CIBR is an NGO in Washington DC and its members include more than 50 radiology departments of universities and 9 companies. Prof. Ma is one of the steering committee members of 9 companies (including GPS).
AuntMinnie, the largest professional website in the imaging industry, interviewed Yuri on TM’s innovative neonatal MRI product
Clients at TM booth
Time Medical Team